Dr. Hai Qi’s group reveals a new mechanism for sexual dimorphic humoral immunity
On December 25, 2019, Dr. Hai Qi’s group at the Tsinghua University Institute for Immunology published a research article entitled“A GPR174-CCL21 module imparts sexual dimorphism to humoral immunity”in Nature. By identifying GPR174 as a new receptor for CCL21 and demonstrating its control of positioning and GC participation of B cells in a sex-dependent manner, this study reveals a mechanism by which B-cell physiology is fine-tuned to impart sexual dimorphism to humoral immunity.
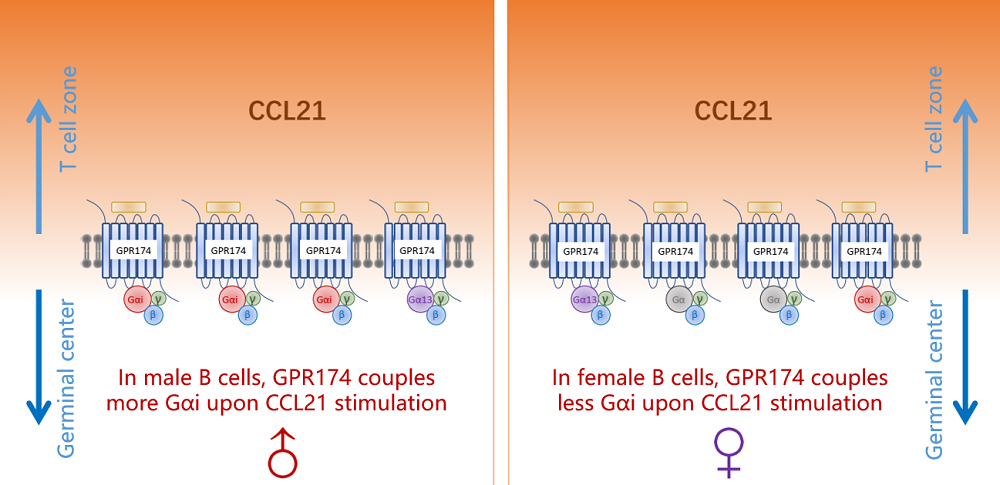
Humoral immune responses to immunization and infection and susceptibilities to antibody-mediated autoimmunity are generally lower in the male1-3. Mechanisms for such sexual dimorphism are not well understood. Here we show that male and female B cells intrinsically differ in forming germinal centers (GC). Antigen-activated male B cells do not as efficiently position themselves in the follicular center, where GCs normally develop. GPR174, an X chromosome-encoded G-protein coupled receptor, suppresses GC formation in male but not female mice. This effect is B-cell intrinsic and correlates with GPR174-enhanced B-cell positioning toward the T-B border and distraction of male but not female B cells from S1PR2-driven follicle-center localization. Biochemical fractionation of conditioned media that induce B-cell migration in a GPR174-dependent manner identifies CCL21 as a GPR174 ligand. In response to CCL21, GPR174 triggers calcium flux and preferentially induces migration of male B cells. Upon CCL21 stimulation, GPR174 is associated with more Gi protein in male than female B cells. Male B cells from orchidectomized mice exhibit impaired GPR174-mediated migration to CCL21, while testosterone treatment rescues this defect. Female B cells from testosterone-treated mice exhibit male-like GPR174-Gi association and GPR174-mediated migration. GPR174 deletion from male B cells permits more efficient positioning toward the follicular center, formation of more abundant GCs, and causes an increased susceptibility to B cell-dependent experimental autoimmune encephalomyelitis.
Dr. Hai Qi is the corresponding author of this article. Ph.D. student Ruozhu Zhao is the first author. Thanks to Dr.Xuyu Zhou (PI, Institute of Microbiology, Chinese Academy of Sciences), Dr.Jianbin Wang (PI, College of Life Sciences, Tsinghua University) and Dr.Tao li (PI, National Center of Biomedical Analysis). This work was funded in part by National Natural Science Foundation of China (grant No. 81621002, 31830023, 81761128019, and 81425011), the Tsinghua-Peking Center for Life Sciences and Beijing Municipal Science & Technology Commission. This work was also funded in part by the Bill & Melinda Gates Foundation and the Howard Hughes Medical Institute. The findings and conclusions within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation or the Howard Hughes Medical Institute.
https://www.nature.com/articles/s41586-019-1873-0